Keywords:
Tecan uses cookies to improve our website. By continuing to browse our website, you accept our cookie policy.
Tecan uses cookies to improve our website. By continuing to browse our website, you accept our cookie policy.
Keywords:
By Severin Heynen
Successful scientific projects build on accurate results from analytical methods that you have confidence in. In regulated situations, they must be backed up by routines and documentation that follow regulatory standards that satisfy the requirements of, for example, the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). Let’s briefly look at qualification and validation of an analytical method and how progress can be made by incorporating integrated analytical solutions.
.png?width=625&name=Picture1%20(1).png)
The chain is only as strong as the weakest link. Ensure that your pipette tips are validated for use in your application.
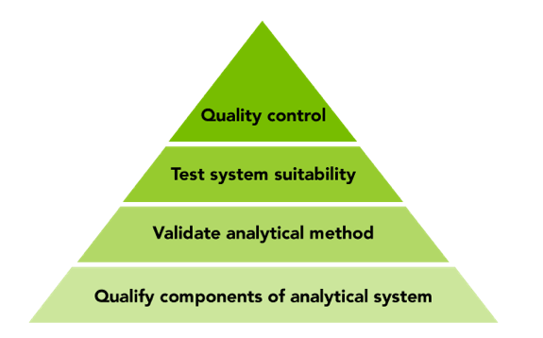
Documented data accuracy builds from the bottom up, starting with qualification, moving on to validation and testing system suitability, and finally QC using known samples or standards.
Securing the accuracy of your data starts with qualifying instruments and components used in the analytical method – for example, liquid handling robots and plate readers. The road toward documented accuracy of analytical methods starts with Installation Qualification and Operational Qualification (IQ/OQ), complemented by Performance Qualification (PQ) if necessary, for testing the system under load. Qualification confirms that the instruments perform as claimed by the vendor, independent of a specific application or sample. Qualification can often be based on the user manual provided.
The IQ indicates that the equipment is installed correctly, and the OQ, run by the vendor or supplier at installation or service interval, confirms that the instrument performs as claimed by verifying against traceable standards. For example, the documentation can show that a liquid handling robot dispenses correctly and that it is properly maintained and calibrated. Missing out the qualification step can lead to significant delays caused by inaccurate results and time-consuming troubleshooting.
The next step is to validate the analytical method using qualified instruments, which may be supplied by different vendors. This can include, for example, specifying the limit of quantitation or detection of sample compounds using a complete system, backed up with calibration standards and SOPs. You can then combine any specific instrument with a specific method and run system suitability tests. This step ensures that the complete system meets the analyst’s expectations under the specific conditions of the tests.
Final QC involves putting together qualified instruments and validated methods to analyze quality control samples or standards and comparing the results with the known composition of the test samples used. Once the analytical method has passed this test you can be confident in the results and that they will be accepted by the regulatory authorities.
Analytical solutions can be constructed using qualified components supplied by different vendors, but the resulting analytical chain is only as strong as the weakest link. Poor performance of just one component (for example a pipette tip) that is not fully compatible with another component can lead to costly delays in reaching final validation. The most efficient way forward is to use components that are already guaranteed to function correctly together and form a truly integrated solution. We will look at the value of integrated solutions in more detail in the next post in this series.
Sign up for the blog to receive updates.

Dr. Severin Heynen joined Tecan in 2015 as a product manager responsible for the disposable tip consumables. In his PhD studies at the University of Zurich, he mainly used biochemical and molecular biology techniques to study the mechanisms of retinal degenerative diseases.