Keywords:
Ion channels regulate many physiological processes, as well as playing a role in many diseases, making them a target for 20 percent of registered drugs. Patch clamping remains the gold standard assay for investigating ion channels, but the manual technique requires patience, and extensive training, and has a low throughput, resulting in ion channels remaining poorly understood.

Sophion Bioscience, in Ballerup, Denmark, was established in 2000, and among its aims was a desire to ‘take the voodoo out of patch clamping’. Since its start-up days as a spin-out from NeuroSearch, its team of dedicated electrophysiologists has expanded to a company of around 50 employees designing automated patch clamping solutions. Rasmus Bjørn Jacobsen, head of the applications development group, explained: “Patch clamping is a direct measurement technique used to investigate the passage of ions across cell ion channels. The whole-cell method uses an electrode-containing glass pipette to create a gigaohm seal with a cell’s membrane. A small amount of suction ruptures the membrane to bring the cytoplasm and pipette solution in direct contact, enabling the electrode to measure currents between 10-12 and 10-9 A passing through the ion channels.”
“Traditional, manual patch clamping requires a high level of skill, and it can take a year’s training to become proficient. Even then, throughput is limited to five to ten assays per day, and each practitioner has their own equipment, which raises concerns about reproducibility. Alternatives to patch clamping involving fluorescent dyes have a much higher throughput, but suff er from low sensitivity and accuracy. With such obstacles to overcome, it made sense to design an automated solution.”
In 2004, the company launched QPatch® – a fully automated patch clamping system – into the market. Göran Mattsson, global product manager for QPatch, said: “The whole assay process is handled by the instrument, from cells to compounds to consumables. The user simply presses ‘Start’ and the experiment runs for up to fi ve hours. This unattended operation is an essential requirement of many pharma companies, who also demand high quality standards and reproducibility. Three quarters of the top 20 pharma companies worldwide are now using a QPatch – or other Sophion products – for drug discovery and safety testing, and it has become the benchmark for cardiac safety.”
Partnering with Tecan allows us to deliver the complete service package, not just a high quality instrument.
In 2004, the company launched QPatch® – a fully automated patch clamping system – into the market. Göran Mattsson, global product manager for QPatch, said: “The whole assay process is handled by the instrument, from cells to compounds to consumables. The user simply presses ‘Start’ and the experiment runs for up to five hours. This unattended operation is an essential requirement of many pharma companies, who also demand high quality standards and reproducibility. Three quarters of the top 20 pharma companies worldwide are now using a QPatch – or other Sophion products – for drug discovery and safety testing, and it has become the benchmark for cardiac safety.”
“The QPatch brings together the accuracy of manual patch clamping with the high throughput of alternative methods, and can make up to 48 recordings in 30 minutes. Training a technician on the instrument takes just two days, and the assay process does not require expert oversight. One person can prepare the cells, another can start the process, and someone else can analyze the results. The built-in security is also an added benefit; the QPatch connects to an Oracle database, which logs all data and is FDA 21 CFR Part 11 compliant. The data cannot be deleted or overwritten, providing reassurance to pharma companies that they can trust the data recorded anywhere in the world.”
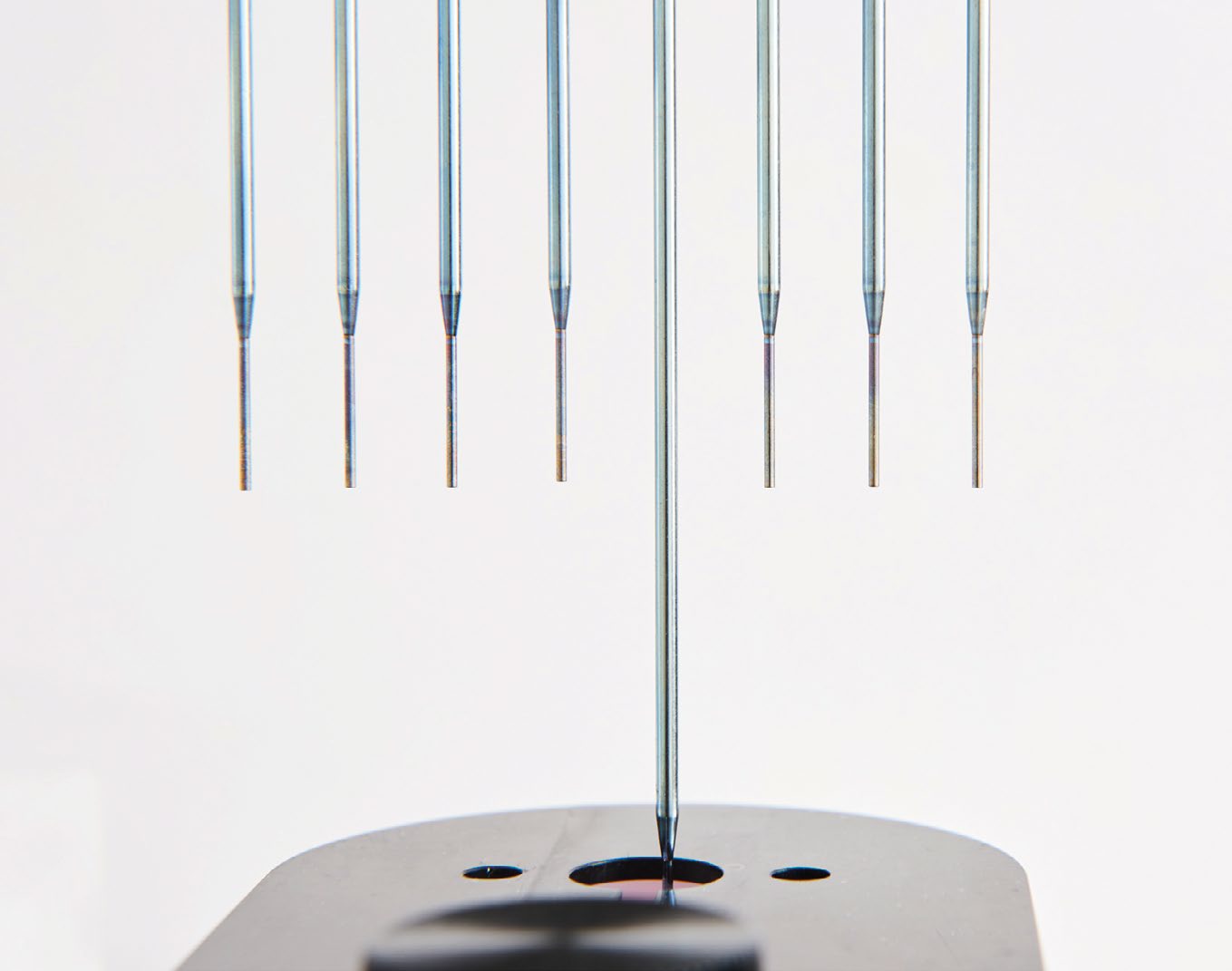
Jørgen Due, head of service, continued: “Patch clamping requires the utmost precision and accuracy, and is extremely sensitive to electrical noise given the tiny currents we are measuring. When designing the QPatch, we looked at what OEM liquid handling solutions were available on the market, and we came across the Sias® Xantus® robotic pipetting platform from Tecan. The Xantus fulfilled our specific needs, and we appreciated its self-contained design; the power and the cables connect to the back end, and everything else – including the pump – resides in the robotic arm. Crucially, the Xantus can switch between multichannel and single channel pipetting, with the option to cherry-pick samples, and we can upgrade the system when a customer requires increased throughput.”
“It’s been great to work with Sias’ in-house engineering team and we collaborated quite closely to develop the QPatch. Not only did they help with the integration of the Xantus robot, they also designed a special titanium oxide coating for the pipette probes to help prevent compounds sticking to them. Sias set the bar for customer service support by providing valuable training to our engineers, and this standard has continued under Tecan. In the future, if we are bringing another product to market, then I’m sure our strong relationship is set to continue. Partnering with Tecan allows us to deliver the complete service package, not just a high quality instrument,” Jørgen concluded.
All Tecan products mentioned are for research use only. Not for use in clinical diagnostics.
To find out more about partnering with Tecan, visit www.tecan.com/partnering
To learn more about Sophion Bioscience, visit www.sophion.com
Keywords:









