California-based company zPREDICTA™ has created a novel technology that reconstructs physiologically-relevant organ-specific human microenvironments that help eliminate the guesswork from drug development. Meaningful drug discovery studies involve complex experiments that are not feasible to perform manually. Automation is the answer, improving accuracy, saving time and reducing the amount of compound used.

A major problem in drug discovery and development is that a high proportion of the drugs reaching clinical trials – around 95 percent – ultimately fail due to a lack of efficacy. In part, this is because non-physiological 2D cell culture and mouse models frequently used in preclinical drug discovery have limited effectiveness as a representation of human diseases. This can result in a poor correlation with the response observed in clinical trials; drugs demonstrating promising results in preclinical studies in physiologicallyirrelevant models may prove ineffective, while compounds that were deprioritized on the basis of a poor performance in mouse models might actually work very well in people. Based on the principle that cures for human diseases require a human-specific discovery and testing environment, zPREDICTA, based in San Jose, USA, developed a novel 3D technology for reconstruction of a physiologically-relevant, organ-specific human microenvironment, the Reconstructed Organ (r-Organ) platform, which mimics the native architecture of human tissues in an organ- and diseasespecific manner. As a result, there is a high correlation between preclinical studies and clinical response.
The r-Organ platform lies at the core of zPREDICTA’s goal to integrate different technologies and automate the various steps in drug development and establish a seamless workflow, from the initial drug screening process through to preclinical and clinical studies. Dr Julia Kirshner, founder and CEO, explained: “Our research involves three-dimensional organ reconstruction within an organspecific extracellular matrix, followed by cell culture under human disease conditions. As this process is very close to what happens in a person, when the drugs are applied to this platform they behave in the same way as they do in a patient, resulting in a high correlation with clinical response.”
Julia continued: “The r-Organ platform is quite versatile – it can handle a wide range of drug types, including small molecules, antibodies, antibody drug conjugates, and CAR-T cells – and the technology is already starting to be used in the pharma industry. We currently off er a fully validated Reconstructed Bone Marrow (r-Bone) for the study of leukemia and multiple myeloma, and are establishing a lung system for the investigation of non-small cell lung cancer. We also have other tissues at different stages of development and validation, including brain, a lymphnode for the study of lymphomas, and mammary gland tissue for breast cancer. These physiologically-relevant tissues are ideal for drug development studies.”
"This [the D300e] has contributed to a four- to five-fold improvement in accuracy."
“When you are performing drug testing studies, multiple steps are involved – dilution, dose-response, combination treatment – to acquire data at different doses and various time points, and the process really needs to be automated. For instance, some larger scale projects have involved a couple of dozen patients, with 240 drugs and a seven-point dose-response curve. That’s anything from a couple of thousand wells through to 10,000 for the entire study, which is a huge undertaking manually. It is critical that there are no transfer errors, but it is very difficult to manually track every well to ensure that the contents are correct. You also need to take variation across the plate – for example, due to evaporation – into consideration by randomization of the well contents. Manual processing is just not feasible. It is incredibly tedious and extremely difficult to do without making mistakes, and that’s why we invested in a D300e Digital Dispenser from Tecan.”
“We’ve had the D300e for about 18 months now, and find it very user friendly and easy to operate. Automation has made a tremendous difference to our workflows. Manual errors, such as missing wells or pipetting into the wrong well, have been eliminated, and we can set the system to shake the plate after dispensing to ensure even distribution and suspension of the drugs in the matrix, preventing the creation of higher dose pockets. This has contributed to a four- to five-fold improvement in accuracy. We save a great deal of time too. Manual set-up that took three and a half hours can be completed in just 15 minutes with the D300e, and the treatment time for 60 plates is reduced from 26 to two and a half hours. Other benefits include an 80 percent decrease in the amount of compound used, and more than a 2,000 times reduction in waste, which will become increasingly important as we scale up our experiments.”
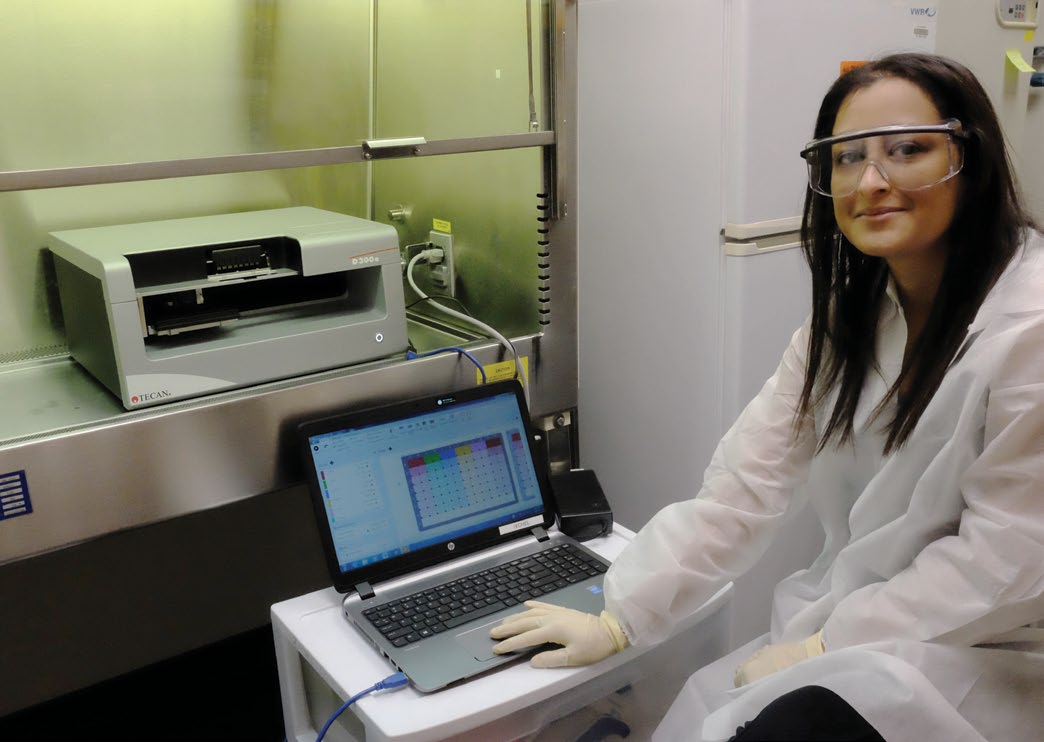
“I really enjoy using the D300e and it’s a pleasure working with Tecan’s representatives, engineers and scientists. The team is very professional and we have established a great relationship,” Julia concluded.
All Tecan products mentioned are for research use only. Not for use in clinical diagnostics.
To find out more about Tecan’s cell biology solutions, visit www.tecan.com/cellbiology
To learn more about zPREDICTA, go to www.zpredicta.com









