Keywords:
By Dr Christian Oberdanner
Achieving reproducibility in a cell-based fluorescence assay can be a real challenge. For example, you might run a very basic experiment to determine the optimal concentration of cell media supplements for your primary cell line. You're looking for differences in proliferation. Starting with a low cell number and a concentration series of media supplements, you use fluorescently-transfected cells to follow the FCS-dependent growth in a 48-well plate over several days by measuring the GFP signal with fluorescence bottom readout. But poor well-to-well and plate-to-plate reproducibility will give a high overall signal variation (increased coefficient of variation, or CV%), which means you may not discern significant differences among the samples. The differences might actually be there, but they are hidden by measurement variation. What should you do?
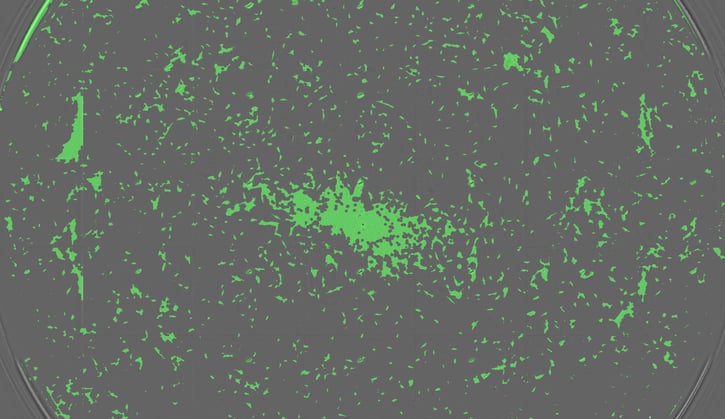
The spread of human fibroblasts (nnHDF) cultured in a single well of a 48-well plate. Many cell types tend to proliferate in a non-uniform manner across the growth surface, so reading the whole well is very important.
It’s a multidimensional challenge
Simply put, getting high quality data from cell-based fluorescence assays means developing a sensitive, reproducible and accurate assay setup and using it to measure signal from as many of the cells in the culture well as possible whilst minimizing background. The challenge is therefore multidimensional, both optically and spatially, and success can be achieved by looking at four main factors that underpin high-performing assays.
Tune in to the right wavelengths
To start with, ensure that the wavelengths you are using for excitation and emission are optimal. If you don’t then you risk getting variable results from low signals and high backgrounds. Optimization means employing the best possible optics to scan through wavelengths for both excitation and emission that match the fluorophore you are using in the cell culture environment. This analysis should take into account any influences from the environment that can shift fluorescence or cause background, such as autofluorescence.
Get to the bottom of the problem
Adherent cell lines are usually analyzed through the bottom of the culture well. This approach ensures higher sensitivity by bringing the cells closer to the detector and also minimizes unspecific signal detection coming from the culture medium in the wells. It also means that measurements can be taken while cell cultures are covered to protect from contamination and evaporation. The key is therefore to use optics that provide focused analysis of the bottom of the well whatever the architecture of the plate you happen to be using.
Don’t miss anything
Many cell types tend to show a non-uniform distribution across the growth surface, i.e. denser growth in the middle of the microplate well and more sparse growth towards the periphery. Measuring fluorescence arbitrarily in the center of the well would therefore give a false impression about the cell density. This can be a real issue when starting with low cell numbers, like the FCS-optimization example we started with. Even 60% confluence can lead to problems. To tackle this problem you need to ensure that you take readings from over the whole surface of the well bottom, whatever the plate format used.
Better interpretation with normalization
Assuming that you have ticked all the boxes above, you should be getting accurate and sensitive data from your cells. But signal is not necessarily equal to cell density. There may be issues with cell health and edge effects that cause signal variation between cells and skew your results. A good way to spot these effects is to normalize your data using confluence level.
Find a reliable research partner
Addressing all the issues above will certainly help you to get the assay reproducibility you need to get reliable data and detect those significant differences in your experiments. The final trick is to choose the instrumentation and software that will help you develop the assays and apply them, quickly and effectively. How that can be achieved will be the subject of the next post in this series.
Keywords:
About the author

Christian Oberdanner
Dr. Christian Oberdanner is Product Manager at Tecan Austria. He studied molecular biology at the University of Salzburg with a strong focus on cell- and tumor biology during his PhD study. Christian started to work for Tecan Austria as external scientific consultant in 2005 and permanently joined the company in 2006. Since then he held the role of an Application Specialist in the research and development department as well as in the sales and marketing department and since 2015 of a Product Manager. Christian’s priority within Tecan is the multimode microplate reader portfolio.











