By Oliver Schmidt
HMGB1 is a key mediator in the immune response to SARS-CoV-2, and increased levels can be an important indicator for COVID-19 understanding and its prognosis. In this final piece in our series, we look at the performance of Tecan’s HMGB1 ELISA kits, and demonstrate the value of total HMGB1 as a clinical biomarker in a wide range of sample types and diseases. These highly sensitive kits are regarded by key opinion leaders to be the gold standard for quantitative HMGB1 analysis, making them the ideal starting point when it comes to measuring HMGB1 in COVID-19 patients.
HMGB1 ELISA tests and COVID-19
HMGB1 plays a key role in many diseases, including COVID-19, as discussed in our first article in this series. However, the accurate measurement of HMGB1 using ELISA used to be fraught with difficulty, mainly due to non-specific activity or cross-reactivity associated with available HMGB1 antibodies. Tecan’s HMGB1 ELISA kit has overcome this major obstacle, and its accuracy and reproducibility have made it the kit of choice in over 1400 publications to date.
Measuring HMGB1 in the immune response to SARS-CoV-2
What do HMGB1 pioneers say about Tecan’s HMGB1 ELISA kits?
Professor Helena Erlandsson Harris, a pioneer in HMGB1 research, found the Tecan HMGB1 ELISA kit to be the most reproducible of all those assessed by her teams, noting: “Perhaps most important is the fact that this kit has become the gold standard within the field, which means you can compare your results with what other groups are publishing”.
A detailed evaluation by Dr. Julia Lehner of the second-generation kit developed by IBL at the Institute of Clinical Chemistry, University Hospital Munich-Grosshadern in Munich, Germany, demonstrated it to be, as she and her co-authors state, “a robust and safe assay, producing reliable quantitative results from serum samples”.5 The kit shows virtually no cross reactivity to HMGB2, which can be a serious confounding factor when measuring HMGB1 using other kits.
Changes in the level of HMGB1 can be readily measured once the normal level has been determined through careful analysis of a relevant population. Many factors influence this level, such as smoking, alcohol intake and diet.1 Studies in many Western countries regard the normal level to be approximately 4 ng/mL, while the Japanese study group used for kit validation had a level of < 1.4 ng/mL.
The Tecan HMGB1 kit can be used on samples from a wide range of body fluids, including serum, plasma, synovial fluid, cerebrospinal fluid and cell culture extracts. The case studies described here illustrate how it has been used to demonstrate the utility of HMGB1 as a valuable biomarker. The kit has also been used in a large number of in vitro studies, and in a range of mammalian species. The Tecan HMGB1 ELISA Kit is CE marked for in vitro diagnostic (IVD) use in Europe but is validated for research use only in the US.
Case Study 1: HMGB1 in serum is an aid for a diagnostic for myocardial infarction
Percutaneous coronary interventions have improved the clinical outcome in patients with a full-blown heart attack caused by the complete blockage of a heart artery (STEMI) or non-ST-elevation myocardial infarction (NSTEMI), but patients with large infarctions and severe left ventricular dysfunction still have a poor prognosis. Myocardial inflammation is one of many causes of chronic heart failure and Professor Martin Andrassy and his colleagues at the University of Heidelberg in Germany were first in showing that increased HMGB1 levels are closely related to infarct extent in patients with acute myocardial infarction.2 These results are outlined in Figure 1.
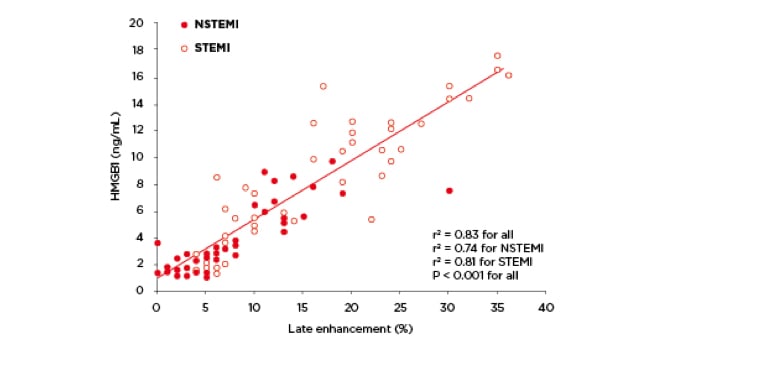 Figure 1: HMGB1 expression was related to infarct extent in patients with ST-elevation myocardial infarction (STEMI) or non-ST-elevation myocardial infarction (NSTEMI). Redrawn from Figure 1(a) in Andrassy et al, 2011.2
Figure 1: HMGB1 expression was related to infarct extent in patients with ST-elevation myocardial infarction (STEMI) or non-ST-elevation myocardial infarction (NSTEMI). Redrawn from Figure 1(a) in Andrassy et al, 2011.2
Case Study 2: HMGB1 in cerebrospinal fluid is a predictive marker for traumatic brain injury research
Traumatic brain injury (TBI) is a significant cause of morbidity and mortality in children in the United States, with over 470,000 emergency department visits annually of children 0–14 years old, leading to 35,000 hospitalizations, and 2,000 deaths. Dr. Alicia Au and her colleagues at the Safar Center for Resuscitation Research and the Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, USA, investigated the value of HMGB1 as a possible predictive marker that could direct effective treatment. They found that raised levels of HMGB1 were indeed associated with poor outcomes in infants and children with TBI, signaling tissue necrosis (Figure 2).3
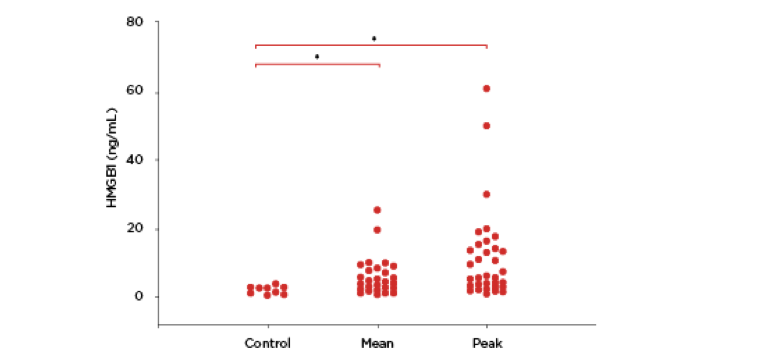 Figure 2: Mean and peak levels of HMGB1 in CSF from patients with TBI were raised compared with controls (*p < 0.05). Redrawn from Figure 1B, Au et al. 2012.3
Figure 2: Mean and peak levels of HMGB1 in CSF from patients with TBI were raised compared with controls (*p < 0.05). Redrawn from Figure 1B, Au et al. 2012.3
Case study 3: HMGB1 in synovial fluid is an independent factor for radiographic severity of osteoarthritis
Osteoarthritis is a common degenerative joint disease and a leading cause of disability in the elderly. It is a chronic inflammatory process, with the knee being a primary site for its development and progression. Zhan-Chun Li and colleagues at the Department of Orthopedic Surgery, Renji Hospital in Shanghai, China compared levels of HMGB1 in synovial fluid taken from the knees with those of HMGB1 in the serum of seventy-eight osteoarthritis patients and thirty controls.4
They were able to conclude that osteoarthritis patients had higher levels of HMGB1 in the knee synovial fluid than controls. These levels also correlated positively with scores based on the Kellgren-Lawrence (KL) grading system for osteoarthritis, and HMGB1 level was an independent factor for radiographic severity of osteoarthritis. In contrast, the researchers did not detect any significant differences in serum levels between patients and controls.4
How can we add the Tecan HMGB1 ELISA kit to our COVID-19 armory?
A major obstacle to the establishment of ELISA tests for HMGB1 has been finding adequate antibodies, such as the one developed and used in the Tecan gold standard kit. The hope for the future of HMGB1 ELISA tests lies in further development of more specific antibodies, particularly since increasing specificity has the potential to enable decreased assay times, higher sensitivity and increased dynamic range of detection.
Since HMGB1 is a major mediator in the immune response, a significant reduction in the current 20h-24h incubation time for the assay would be advantageous for clinical research and diagnostics applications—particularly in the case of diseases such as COVID-19, where progression of severity can be extremely rapid.
With these needs in mind, researchers at Tecan have developed a new generation of HMGB1 ELISA. The new generation HMGB1 Express ELISA (cat. number 30164033) gives comparable results to the current kit (cat. number ST51011) in as little as one-sixth of the time and is already available for clinical research (USA) or diagnostic use* (Europe).
The main features of the kit are:
- New monoclonal anti-HMGB1 pre-coated to microtiter wells
- Significantly reduced assay time: 4 hours (currently ~24h)
- Larger dynamic range, up to ~2.0 OD (currently 1 – 1.5 OD)
- Wide measuring range (0.89 – 80 ng/mL)
- Good correlation to current HMGB1 ELISA data
*The product HMGB1 express ELISA may not be available on your territories, consult your local Tecan office.
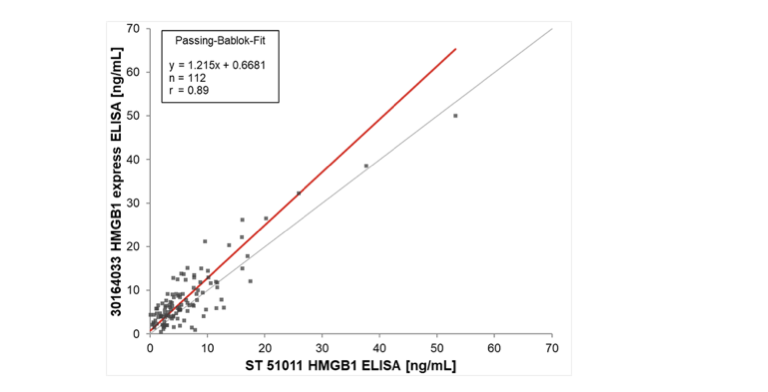 Figure 3: Correlation of results between HMGB1 express ELISA kit and existing HMGB1 ELISA kit
Figure 3: Correlation of results between HMGB1 express ELISA kit and existing HMGB1 ELISA kit
We hope this series has brought you some fresh insights into the crucial role of HMGB1 in the immune response to disease, especially as we learn more about its involvement in SARS-CoV-2 infection and progression in severe cases of COVID-19. To facilitate the evaluation and validation of the new HMGB1 Express ELISA kit in your lab, particularly if you are already using the current kit, please contact your local Tecan specialist directly.
References
1. Wang, X., Bu, H. F., Zhong, W., Asai, A., Zhou, Z., & Tan, X. D. (2013). MFG-E8 and HMGB1 are involved in the mechanism underlying alcohol-induced impairment of macrophage efferocytosis. Molecular medicine (Cambridge, Mass.), 19(1), 170–182.
PubMed ID: https://pubmed.ncbi.nlm.nih.gov/23552724/
DOI: https://doi.org/10.2119/molmed.2012.00260
2. Andrassy, M., Volz, H. C., Riedle, N., Gitsioudis, G., Seidel, C., Laohachewin, D., Zankl, A. R., Kaya, Z., Bierhaus, A., Giannitsis, E., Katus, H. A., & Korosoglou, G. (2011). HMGB1 as a predictor of infarct transmurality and functional recovery in patients with myocardial infarction. Journal of internal medicine, 270(3), 245–253.
PubMed ID: https://pubmed.ncbi.nlm.nih.gov/21362071/
DOI: https://doi.org/10.1111/j.1365-2796.2011.02369.x
3. Au, A. K., Aneja, R. K., Bell, M. J., Bayir, H., Feldman, K., Adelson, P. D., Fink, E. L., Kochanek, P. M., & Clark, R. S. (2012). Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. Journal of neurotrauma, 29(11), 2013–2021.
PubMed ID: https://pubmed.ncbi.nlm.nih.gov/22540160/
DOI:https://doi.org/10.1089/neu.2011.2171
4. Li, Z. C., Cheng, G. Q., Hu, K. Z., Li, M. Q., Zang, W. P., Dong, Y. Q., Wang, W. L., & Liu, Z. D. (2011). Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clinical and investigative medicine. Medecine clinique et experimentale, 34(5), E298.
PubMed: https://pubmed.ncbi.nlm.nih.gov/21968272/
DOI: https://doi.org/10.25011/cim.v34i5.15673
5. Lehner J, Wittwer C, Fersching D, Siegele B, Holdenrieder S, Stoetzer OJ. Methodological and preanalytical evaluation of an HMGB1 immunoassay. Anticancer Res. 2012 May;32(5):2059-62. PMID: 22593488.
PubMed: https://pubmed.ncbi.nlm.nih.gov/22593488/
About the author

Oliver Schmidt
Oliver Schmidt is product manager for research reagents, neurodegeneration, and bone and mineral products at TECAN-IBL in Hamburg with more than 10 years’ experience in bringing these products to the Life Science and academic community. He studied Environmental Engineering at the Technical University of Hamburg-Harburg and joined TECAN-IBL in 2005.











