Keywords:
By Nastya Yeska
The accurate measurement of female hormone levels is at the very core of women’s reproductive health and general wellbeing, whether searching for potential causes of infertility, or treating debilitating premenstrual or menopausal symptoms. These tests were traditionally blood-based. However, saliva-based testing is now recognized as a highly accurate and painless alternative. In our final article in this series, we examine some of the different saliva-based testing technologies and protocols available for measuring and monitoring female hormone imbalance.

Saliva diagnostics are set to become increasingly routine and represent a significant step forward for reliable female hormone testing.
So, you want to do saliva-based testing?
Saliva-based diagnostics are revolutionizing the field of personalized medicine since they can be used to evaluate many different physiological conditions. This includes tracing the progression of disease, and monitoring the efficacy of therapies, as well as measuring female hormones, our focus here.1
Once you are satisfied that you can obtain equivalent results from saliva and blood, you also need to be sure that your assay technology is scientifically credible as a basis for consultation and treatment, and that your tests will be economically viable enough to warrant investment, even in the absence of routine public healthcare or insurance cover.
As a service provider you may also be keen to become part of an infrastructure that contributes towards the universal standardization of saliva testing, as it moves into the mainstream.
Are my tests scientifically credible?
Let us break down the answer to this question by considering the three major parts to the testing workflow: sample collection, the assay itself, and how to interpret the results.
Sample collection: a stimulating subject
We saw in our last article that sample collection involves the careful choice of collection method, and the avoidance of devices which may negatively affect the result, such as cotton-based swabs.2,3 The suitability of the collection method for the key analyte has often not been considered at all in peer-reviewed papers, and the lack of standardized sampling procedures continues to make it difficult to compare results from different laboratories. This has been extensively reviewed by Bellagambi et al., with the pros and cons for different collection methods, including whole saliva versus glandular, and stimulated versus unstimulated sampling.4 Because so many different factors can influence salivary secretion and composition, a precise standard for saliva collection must be established to obtain reliable and comparable data for female hormone testing.
Saliva assays: to MS or not MS?
Mass spectrometry (LC-MS/MS) is the reference method for steroid hormone quantification. However, it represents a significant financial and training investment, which may not be available to all laboratories. It would therefore help the acceptance and adoption of saliva-based assays if more accessible technologies were developed that could be correlated to LC-MS/MS.
Other assay needs would be:
- low sample volumes, ideally around 50 µL per assay, to allow a panel of hormones to be tested for a given saliva sample
- ability to refer to defined reference values throughout the menstrual cycle for women of reproductive age, or to “normal” values for postmenopausal women
- whilst not essential, standardization of the sample diluent so as to have the same diluent for all the different assays would offer additional convenience, as opposed to having a different one for each assay
Figure 1 demonstrates one example of the strong direct correlation between ELISA and LC-MS/MS results. Provided that the results from a specific ELISA or luminescence immunoassay test for female hormones have been shown to correlate to LC-MS/MS results in this way, the user can be confident of their result, with the caveat that the different saliva samples have been collected from the patient using a recommended, standardized protocol.
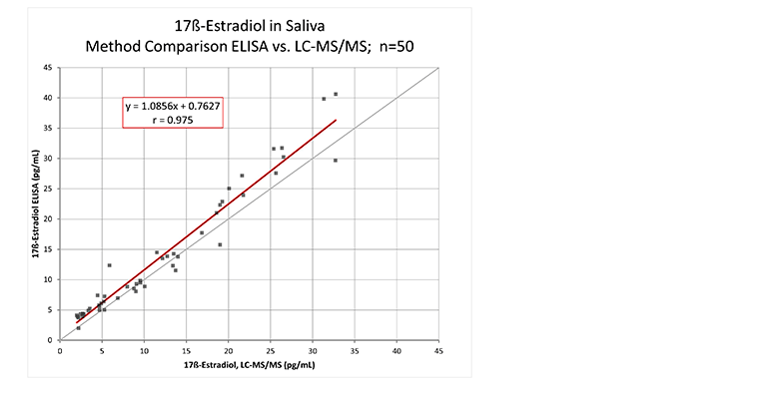
Figure 1. Correlation of Tecan 17ß-Estradiol Saliva ELISA to the LC-MS/MS reference method. ELISA testing was performed using 17β-Estradiol Saliva ELISA kit (Tecan), following the protocol provided in the product literature.
Luminescence assays offer an alternative sensitive detection method, due to enhanced signal multiplication and amplification, in a process whereby an enzyme converts a substrate to a reaction product that emits photons of light instead of developing a visible color.
How do I interpret my results?
The results from a saliva-based assay are not an end unto themselves; they must be correlated to other clinical observations and diagnostic tests to create a holistic picture that will help shape the patient’s follow-up and potential future treatment. The reasons for testing for female hormone imbalance in the first place need to be considered, and the results compared with “normal” reference ranges.
Take the 17ß-Estradiol Saliva ELISA test as an example. Even before starting to work with patient samples, a laboratory must establish its own range of “normal” values. To establish a normal range for this particular test, a study could be performed with pre-menopausal women not using contraceptives.
For the study described in this example, saliva samples were collected five times during a period of 2 hours after awakening, covering the whole menstrual cycle, for a maximum of 30 days. Samples were pooled per day and the estradiol concentration was measured to obtain a daily value throughout the menstrual cycle. Figure 2 shows the results of the study.
.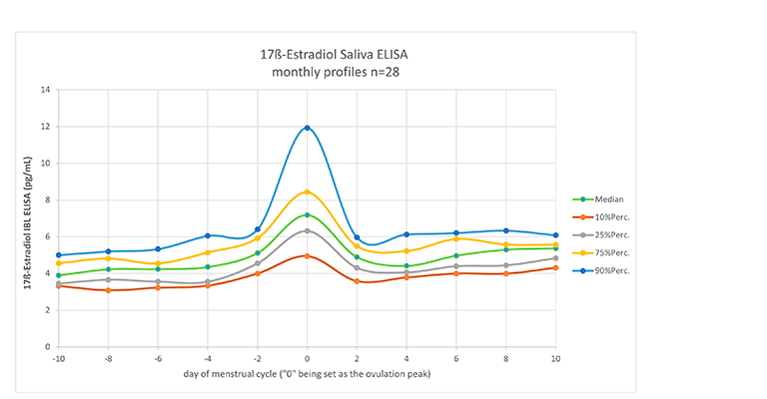
Figure 2: Daily values of 17ß-Estradiol (pg/mL) in saliva samples from 28 women over 1 month. ELISA testing was performed using 17β-Estradiol Saliva ELISA kit (Tecan), following the protocol provided in the product literature.
This shows evidence of the scientific validity of saliva testing for 17ß-Estradiol: as found with blood samples, the ovulation peak is clearly visible. Such a graph can then form a basis for comparison with patient samples, to see if the levels of 17ß-Estradiol fall outside these norms, which could signal a potential reason for infertility, or a move towards menopause. It should be emphasized that all patient hormone test results obtained in this way must be correlated to other clinical observations and diagnostic tests before any clinical decisions are made regarding follow-up treatment: they are an indicator only, and are not be taken in isolation.
Which female hormone tests are most economically viable?
The final answer to this question will depend on the individual lab, with factors as diverse as the local need for testing, the existing infrastructure, and whether public or private funding is available. However, it should also be remembered that we are looking at a bigger picture here: the start of a journey towards hormone balance at a significant point in a woman’s life, whether she is suffering from debilitating menstrual problems, is trying to conceive a much-wanted child, or to ride out a tough menopause. Hence, the cost of a hormone test at this time is likely be overtaken rapidly by far more expensive consultations and follow-up treatments, such as in vitro fertilization (IVF) or hormone replacement therapy (HRT).
Having said that, manual ELISA assays will always be the most economically viable, over automated ELISA, and ELISA will be cheaper than LC-MS/MS. A small community start-up lab could very easily set themselves up by simply buying female hormone saliva ELISA tests, together with a suitable plate reader, provided that the saliva sample collection methods are standardized, validated and calibrated with other labs using similar assays.
As outlined in our previous article, whole saliva testing is also likely to be more economical than blood-based testing, at least in terms of the initial sample collection, since there is no need for phlebotomist expertise, although staff training in sample collection and assay is desirable, to eliminate as many variables as possible (see below).
At the other end of the scale, you might already have or want to invest in an automated workflow for female hormone testing, with robotic automation of microtiter plate assays, linked to an automated plate reader. You might simply be adding “a few more ELISA tests” to your portfolio. Alternatively, you could be starting out with female hormone testing, but with a budget that needs to stretch to other types of automated tests too. Since saliva can be used to measure many other biomarkers, you can easily capitalize on the investment that you put into developing the infrastructure and automation by expanding your saliva testing workflow to include other types of steroids.6
Are my assays sufficiently standardized?
Whilst significant efforts have been made to standardize blood-based hormone testing, standardization and routine implementation of saliva-based testing still has some way to go, being relatively new on the block as a credible way to measure female hormones, even if the ability to test saliva per se has been around for decades. As concluded by Bellagambi et al., saliva analysis as a routine approach in a clinical setting is still hindered by the absence of standardized procedures and analytical information.4
This contrasts with blood protocols, which already have a Centers for Disease Control and Prevention (CDC) Laboratory/Manufacturer Hormone Standardization (HoSt) Program that helps laboratories with lab-developed tests and assay manufacturers with calibration, by assessing the assay performance of the lab or assay manufacturer throughout an entire year. All participants remain anonymous throughout the validation process and are only publicized upon successful completion of the program. The CDC has a series of certified procedures associated with this, one of which can be found here for estradiol.5
Attempts are being made to share salivary data between laboratories with this aim in mind, and several companies are working towards standardization programs. One such example is Tecan’s Quality Assessment Scheme, established back in 2005, since at the time there was no standard available for steroids in saliva.
The Tecan Quality Assessment Scheme is conducted twice a year with an assessment panel that includes cortisol, testosterone, estradiol, progesterone and DHEA in various concentrations. Individual participants submit their results for statistical analysis and receive a personalized QAS result sheet following completion of the assessment. Confidentiality is assured using an ID number known only to the participant and to Tecan.
How much training will my staff need?
A vital and often overlooked aspect of universal standardization is staff training. Given that we are not yet at the same level of standardization with saliva diagnostics as we are with blood-based diagnostics, the different variables in the protocols need to be addressed consistently in training, to ensure that results are comparable from operator-to-operator and lab-to-lab.
As an example, a good training course might include, but not be limited to:
- brief background to the steroids being assayed
- the dynamic nature of steroid secretion
- protocol for collection of saliva samples, including patient education
- effects of blood contamination
- volume of saliva needed
- effect of pretreatment of saliva (freezing, thawing, centrifugation)
- validation and evaluation of the chosen analytical method (sensitivity, specificity, reproducibility, reference values, usefulness of diagnostic method for the intended medical purpose)
- practice run of the assays, and comparison with existing data
It is good practice to have a regular staff training program for saliva testing, and this is something Tecan can help you plan, whether on-site or over the phone.
Setting up saliva-based testing in your lab
In this series, we have explored and overcome some major theoretical and practical obstacles to setting up saliva-based female hormone testing. The key to successful saliva diagnostics is standardization. To this end, Tecan offers a comprehensive female hormone immunoassay portfolio with pre-standardized sample collection and assay protocols. Saliva-based testing has come of age, and is now a recognized reference for measuring female hormone imbalance: are you ready to try it?
1. Zhang, A., Sun, H., Wang, P., & Wang, X. (2013). Salivary proteomics in biomedical research. Clinica chimica acta; international journal of clinical chemistry, 415, 261–265. https://pubmed.ncbi.nlm.nih.gov/23146870/ https://doi.org/10.1016/j.cca.2012.11.001References
- 2. Lewis John G. (2006). Steroid Analysis in Saliva: An overview. Clin Biochem Rev Vol 27, 139-146.
PubMed ID: https://pubmed.ncbi.nlm.nih.gov/17268582/
3. Shirtcliff, E.A., Granger, D.A., Schwartz, E., & Curran, M.J. (2001). Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology, 26:165–73.
PubMed ID: https://pubmed.ncbi.nlm.nih.gov/11087962/
DOI:https://doi.org/10.1016/s0306-4530(00)00042-1
4. Bellagambi, F.G., Lomonaco, T., Salvo, P., Vivaldi F., Hangouet, M., Ghimenti, S., Biagini, D., Di Francesco, F. & Fuoco, R. & Errachid, A. (2020). Saliva sampling: Methods and devices. An overview. Trends in Analytical Chemistry. Elsevier. Volume 124, March 2020, 115781
Elsevier ID:
https://www.sciencedirect.com/science/article/abs/pii/S0165993619304182?via%3Dihub
DOI: https://doi.org/10.1016/j.trac.2019.115781
6. Yoshizawa, J. M., Schafer, C. A., Schafer, J. J., Farrell, J. J., Paster, B. J., & Wong, D. T. (2013). Salivary biomarkers: toward future clinical and diagnostic utilities. Clinical microbiology reviews, 26(4), 781–791. PubMed ID:https://pubmed.ncbi.nlm.nih.gov/24092855/
DOI: https://doi.org/10.1128/CMR.00021-13
Keywords:
About the author

Nastya Yeska
Nastya Yeska joined Tecan in 2017 as a product manager responsible for the Complementary medicine product portfolio, including Food intolerance and Saliva diagnostics. Nastya is part of Global Reagent Marketing & Support Department responsible for delivering ELISA solutions. In 2021, she changed her role to Integrated Marketing Manager.











