Tecan uses cookies to improve our website. By continuing to browse our website, you accept our cookie policy.
Tecan uses cookies to improve our website. By continuing to browse our website, you accept our cookie policy.

Mitosis plays an essential role in growth and cellular replacement, and is often dysregulated in cancers, making the process of therapeutic interest. Researchers at the University of North Carolina at Chapel Hill (UNC Chapel Hill) are developing novel cell imaging tools to help them classify key components of mitosis and identify new therapeutic targets.
Mitosis is the stage of the cell cycle where replicated chromosomes divide into two new nuclei, giving rise to genetically identical cells. In healthy systems, this process only occurs when we are growing, or need to replace old or damaged cells. However, cells grow and divide uncontrollably in cancers. Therefore, fully understanding which molecules control mitosis, as well as what stage of the process they affect, is of interest for the identification of new therapeutic targets. Nicholas Brown, Assistant Professor in Pharmacology at the UNC Chapel Hill School of Medicine, explained: “The process of mitosis is controlled by a signaling cascade called the spindle assembly checkpoint, at the heart of which is the anaphase-promoting complex (APC). The APC functions as a ubiquitin ligase, adding ubiquitin chains to macromolecules to target them for proteasome degradation and move the cell cycle forward. During normal cell function, the spindle assembly checkpoint uses a variety of ‘silencing factors’ to inhibit the activity of the APC – preventing progression of the cell cycle – but these inhibitory signals are disrupted during cancers, leading to uncontrolled proliferation.”
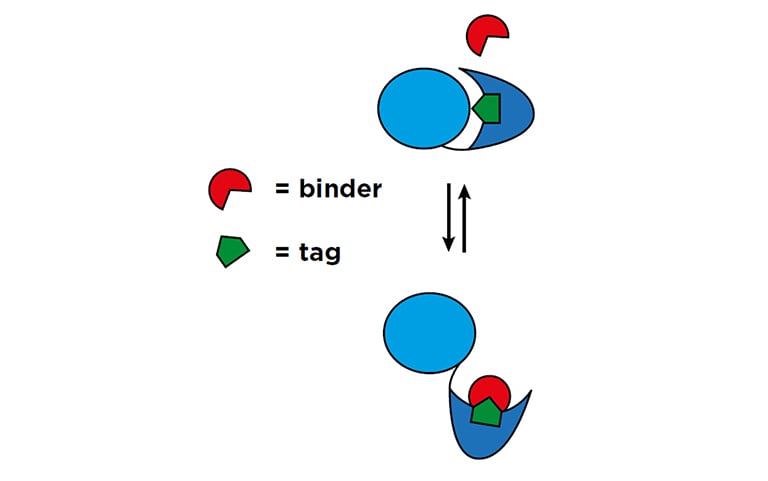 Figure 1: Schematic of the binder/tag approach1
Figure 1: Schematic of the binder/tag approach1
 Nicholas Brown, Assistant Professor in Pharmacology
Nicholas Brown, Assistant Professor in Pharmacology
The lab’s aim is to follow this complex signaling cascade at the molecular level, which requires the development of novel fluorescence microscopy tools for high content screening. Fortunately, the group was able to draw on expertise from within the department, as Klaus Hahn, Thurman Distinguished Professor of Pharmacology, explained: “My lab focuses on building molecules that are used for a wide range of imaging applications in biosensors, optogenetics and chemogenetics. We use them ourselves to study immune cells and motility, but my lab is probably best known for the application of these tools in other labs. Nick therefore approached me with what sounded like a really fun and exciting application of these molecules. He had this exquisitely detailed cellular system, and wanted to ask molecule level questions, such as ‘which conformational changes are occurring?’, ‘how are the different groups abutting each other?’ or ‘what do they sit on?’. This simply isn’t something you normally do in live cell imaging – it is tough to go into that kind of detail in a live cell. But as a chemist, these are very interesting questions to study.”
The Spark Cyto has removed a lot of the tedium from the workflow, so the people in the lab love it.
The joint UNC Chapel Hill team has developed a unique fluorescencebased ‘binder/tag’ approach capable of reporting the conformational changes of individual molecules in live cells (Figure 1). The advantage of this system is that only a very short, seven-peptide tag is inserted into the protein of interest, minimizing the risk of disruption to normal protein or cellular function. The team is now developing and optimizing cell lines with a range of tagged proteins for high content screening. Klaus commented: “Traditionally, optimizing expression of biosensor molecules in live cells for imaging applications is a very tedious process. This means multiple iterations and rounds of cell growth under different conditions. While this is the bread and butter of biosensor development, it is normally quite time consuming. Fortunately, just before starting this project, we were approached by Tecan with the offer to test the Spark® Cyto plate reader with live cell imaging. The company was interested in exploring new ways of using the system, and we were ready with some pretty unique applications here! The Spark Cyto is ideal for this type of biosensor development, massively speeding up the optimization process. It offers the sensitivity and precision required to detect even low output in vitro fluorescent signals rapidly in a 384-well microplate format – something we could not achieve with many other high content platforms. The Spark Cyto has removed a lot of the tedium from the workflow, so the people in the lab love it.”
 The UNC Chapel Hill team (NOTE: Photo taken prior to social distancing)
The UNC Chapel Hill team (NOTE: Photo taken prior to social distancing)
“I’ve used Tecan plate readers since I was a graduate student, and I’ve always liked the brand, so it was a good match,” Nicholas added. “This project is still at the test stage, so we’re performing in vitro screening, purifying all the different tagged assemblies, and making sure that they behave the way we want them to. Once we’ve developed this, we’re planning on moving to in vivo testing using our Tecan system. One of the other things that we’re using it for is monitoring the phases of the cell cycle using a separate set of fluorescent reporters. This obviously requires much longer time periods, as we’re following the whole cell cycle, so the Spark Cyto’s ability to incubate cells and provide quantitative imaging over a full time course is invaluable.”
1) Liu, B et al. Biosensors based on peptide exposure show single molecule conformations in live cells. Cell, 2021, 184, 5670-5685.
To find out more about Tecan’s Spark Cyto, visit lifesciences.tecan.com/sparkcyto
To learn more about the work of the Nicholas G. Brown Lab, go to www.med.unc.edu/pharm/ brownlab
To learn about the Hahn lab and some of the imaging tools discussed, visit www.hahnlab.com