Tecan uses cookies to improve our website. By continuing to browse our website, you accept our cookie policy.
Tecan uses cookies to improve our website. By continuing to browse our website, you accept our cookie policy.

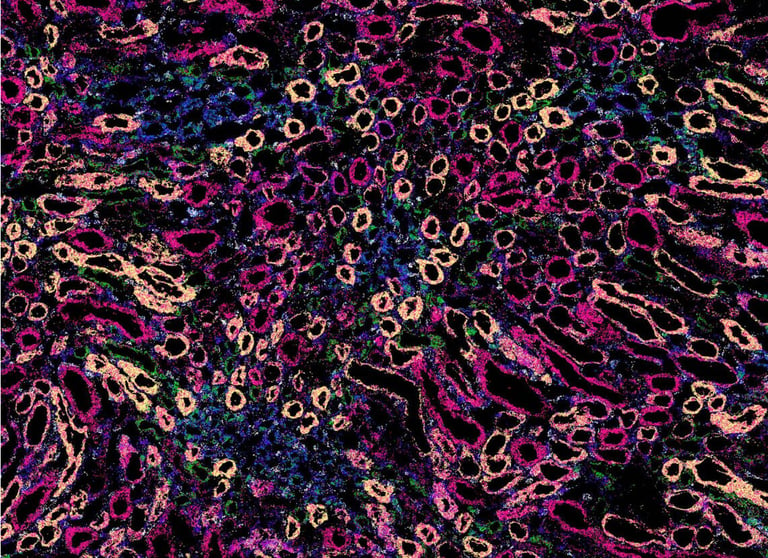
Spatial genomics is a rapidly growing field, allowing researchers to explore gene expression in the context of tissue location. Vizgen has developed the MERSCOPE® Platform, the first commercial high multiplexing, high resolution in situ solution to combine single-cell and spatial genomics analysis. Powered by MERFISH technology, this system not only enables the visualization of gene expression, but also where – and to what abundance – genes are expressed in tissues.
Gaining insights into the positional context of cells relative to one another is key to understanding the complex interactions in tissue biology. Bulk and single-cell sequencing can simultaneously profile a multitude of cells on a transcriptomic level, but these methods require breakdown of the intact tissue to access and sort individual cells. This prevents comprehensive characterization of a cell while also knowing its exact arrangement within the tissue. Spatial transcriptomics company Vizgen, based in Cambridge, Massachusetts (USA), relies on MERFISH (multiplexed error-robust fluorescence in situ hybridization) technology to overcome this challenge, using in situ spatial transcriptomics to enable the quantification of hundreds of gene species at single-cell resolution and in the context of tissue location.

"Tecan's application programming interface and library offered everything we needed for straightforward full integration into MERSCOPE, and we know that the Cavro XC pump will deliver the reliability we need."
For more information about Vizgen, visit www.vizgen.com
The company’s MERSCOPE Platform takes advantage of MERFISH technology to combine single-cell and spatial genomics analysis, providing not just multiplexing capabilities, but also more sensitivity and specificity. Global Instrument Product Manager Robert Mathis explained: “MERFISH is a versatile technology that allows researchers to look at a large number of gene targets – currently up to 500 – on the MERSCOPE Platform with high specificity and sensitivity. Cleverly designed, error-robust barcodes make it possible to efficiently distinguish between targets and correct readout errors, if detected. The technology has many applications, including cell atlasing, where researchers aim to characterize gene expression in both normal and diseased tissue. It enables researchers to understand which gene expression networks are active and inactive, and what is upregulated and downregulated, in different cellular and disease states. We have demonstrated the value and flexibility of MERFISH technology in a variety of applications, including characterization of the molecular homogeneity of the mouse brain and illumination of the tumor microenvironment,1,2 while other researchers in the field have generated publications across disciplines such as neurology, oncology, immunology, infectious disease and more.”
Robert continued: “The workflow is similar to single molecule FISH. Tissue samples are sectioned and placed on our proprietary MERSCOPE Slide – a cover slip-like slide designed to deliver optimum optical clarity. The order of workflow operations varies slightly depending on the sample type – fresh frozen, cell culture or FFPE – but the major steps are all the same: sectioning, hybridization, gel embedding and clearing, followed by MERFISH imaging. The clearing process involves digesting away the tissue components to reduce background autofluorescence while retaining the transcripts. This is unique to MERFISH and MERSCOPE, and the lower background fluorescence supports robust detection of target transcripts.”
“Following sample preparation, the slide is loaded into the MERSCOPE instrument’s flow chamber, and the system automates the entire imaging and analysis process, including cyclic rounds of hybridization with reporter probes. A low magnification (10x) overview showing a mosaic of the whole tissue section is presented to the user, from which they can select specific areas or the full tissue for imaging. Up to one square centimeter can be imaged in total – either as one contiguous area or different regions – then the data undergo automatic cell segmentation and transcript analysis. The sample preparation workflow in general takes about three to four days, although much of that time is incubations; actual hands-on time is probably three to four hours. The imaging time is about 24 to 30 hours, depending on the size of the panel, with a further 24 to 30 hours of analysis time. Throughput is maximized by loading the next sample to be imaged onto the platform while analysis of the previous run completes.”
The success of any automated system depends on reliable, accurate and precise pumping technology, and Vizgen chose the Cavro® XCalibur (XC) Pump. Shawn Wang, Engineering Manager at Vizgen explained: “The Cavro XC Pump lies at the heart of the MERSCOPE fluidics system, drawing liquid from the reagents cartridge into the fluid chamber, and subsequently to waste. It is typically used to move volumes from tens of microliters up to a few milliliters at each stage, with imaging performed in between the fluidic steps.”
“When choosing a pump for MERSCOPE, we were looking for precision and repeatability, and to partner with a credible OEM vendor, such as Tecan. The Cavro XC Pump is one of the few options from major vendors that met our specifications, and our staff also have a history with Tecan, including past positive experiences of its products. Tecan’s application programming interface and library offered everything we needed for straightforward full integration into MERSCOPE, and we know that the pump will deliver the reliability we need,” concluded Shawn.
1. Vizgen. Application note: Distinguish the molecular and cellular heterogeneity of the mouse brain with the MERSCOPE PanNeuro Cell Type Predesigned Gene Panel.
https://info.vizgen.com/resource-download-panneuro-panel-app-note
2. Vizgen. Application note: Illuminate the tumor microenvironment and understand oncological heterogeneity with the MERSCOPE PanCancer Pathways Panel for Human Tissue.
https://vizgen.com/resources/illuminate-the-tumor-microenvironment-and-understand-oncological-heterogeneity-with-themerscope-pancancer-pathways-panel-for-human-tissue-application-note
To find out more about Tecan's OEM liquid handling components, go to www.tecan.com/cavro