By Kevin Moore
In the pharmaceutical industry, stem cells play a growing role in all phases of drug discovery, from disease modeling and early target discovery to their use in developing innovative cell therapies. Increasingly, a major development impact factor for stem cells is their capacity to serve as a self-renewing, sustainable source of differentiated cell models to support predictive toxicity testing in early stage drug discovery.
 Tecan will address an important technology aspect of using stem cells for drug discovery an in upcoming webinar on May 22nd 2018. This informative webinar will describe and discuss the use of automation for high-throughput screening to enable unattended stem cell culture and differentiation to produce high quality cardiomyocytes.
Tecan will address an important technology aspect of using stem cells for drug discovery an in upcoming webinar on May 22nd 2018. This informative webinar will describe and discuss the use of automation for high-throughput screening to enable unattended stem cell culture and differentiation to produce high quality cardiomyocytes.
Stem cell technology: expanding the drug discovery toolbox
Stem cells are becoming increasingly valuable in drug discovery and development based on their role in creating more physiologically relevant disease models for basic biological research and novel drug target discovery, their use in compound library screening, and their advantages in preclinical predictive toxicology testing.¹,²
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), which are derived from adult somatic cells, are self-renewing and pluripotent cells, capable of differentiating into virtually all human cell lineages. These include liver, brain, and heart cells, which are all beginning to play an important role in in vitro pre-clinical predictive toxicity testing.
Using stem cells to detect drug cardiotoxicity earlier
Failure of investigational drugs in late-stage clinical development continues to be a challenging and costly problem for the pharmaceutical industry. A recent study of 640 novel therapeutics found that more than half (54%) failed in clinical development, most (57%) due to insufficient efficacy, and a sizable proportion (17%) due to safety concerns.³
The earlier in the drug development process that these risks can accurately be assessed, the less impact on human health and the financial risk associated with taking a compound forward into clinical testing. The use of stem cell-derived human cell lines in preclinical toxicity testing offers the promise of detecting potentially harmful on- and off-target effects of drug candidates before these compounds progress to clinical trials, helping to reduce attrition rates, costly program failures and post-market withdrawals. If adverse effects can be detected early enough, there may be time for cost-efficient modification of the drug to minimize its toxicity.
Cardiotoxicity is one of the top three causes of post-marketing drug withdrawals due to adverse reactions.⁴ Rapid advances are being made in the use of hiPSC-derived cardiomyocytes to test drug candidates for their potential to cause structural or electrophysiological changes that can adversely affect myocardial contractility.⁵ In a comprehensive review article, authors from the U.S. Food and Drug Administration (FDA) discuss the advantages of using hiPSC-derived cardiomyocytes to assess drug-induced structural cariotoxicity.⁵ They emphasize the renewable and readily scalable nature of stem cells and the benefits of testing directly in human cardiomyocytes rather than having to translate findings from toxicity studies conducted in animal models or animal cell lines.
They conclude that high throughput screening (HTS) using a hiPSC-cardiomyocyte model is a “feasible approach to assess potential contractile and structural cardiotoxicity in early phase drug development,” and they support combining structural and electrophysiological tests on the same platform.
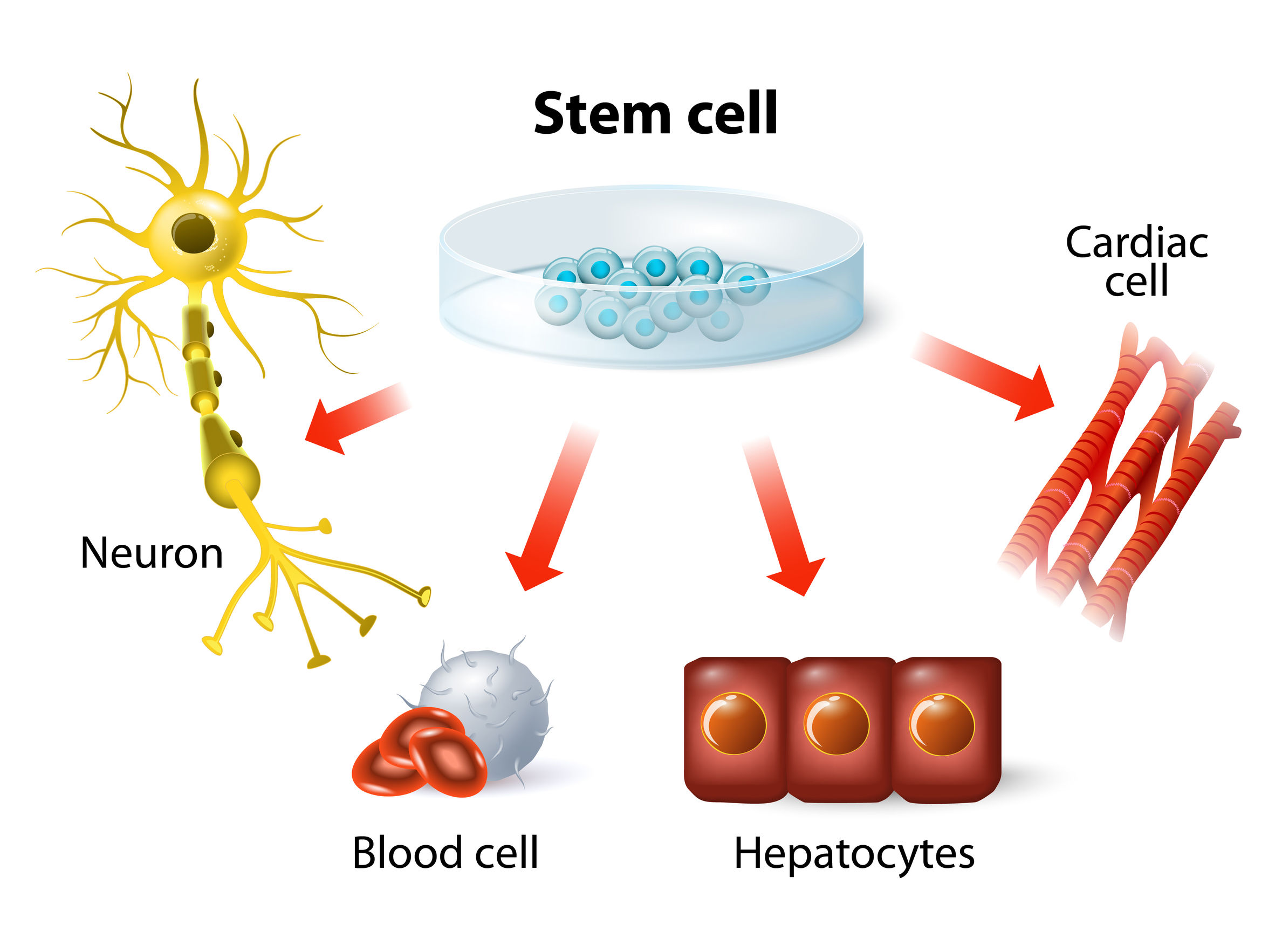
Increasingly stem cells are being used as a self-renewing, sustainable source of cardiomyocytes and other cell models for earlier predictive toxicology testing in pharmaceutical industry drug discovery.
The challenges of working with stem cells
Stem cells are highly sensitive to variability in their environment and their handling, and they can be tedious and tricky to work with. Nurturing stem cells to grow and reproduce in culture is a bit of an art as much as a science.
Even more so are the protocols involved in guiding stem cells down a particular differentiation pathway to form the intended cell type. IPSC differentiation processes require close monitoring of the cells in culture, and the addition of precise amounts of various growth factors and other biological and chemical stimulants at key time points.
Any inconsistencies in how these protocols are carried out — perhaps due to a change in technicians and how they perform these functions — can affect the resulting cardiomyocytes and how they might behave in subsequent toxicity testing.
Automated liquid handling and cell processing technologies can minimize the variability in growing and differentiating stem cells. They can also remove much of the tedious, time-consuming, and labor and cost-intensive aspects of working with stem cells and stem cell-derived models. These benefits become even greater as methods are scaled up to meet the demands of large-scale stem cell applications. High-throughput automation solutions are essential in enabling stem cell-derived models, such as hiPSC-derived cardiomyocytes, to be applied as a pharma industry standard for earlier predictive toxicity testing in drug discovery.
“We used the Tecan Fluent platform to run unattended iPSC differentiation, culturing, and assay protocols over 12 days as part of our routine testing for compound cardiotoxicity during a lead optimization program.”
—Dr. Philip Gribbon, Assistant Head of Department, Fraunhofer Institute of Molecular Biology and Applied Ecology (IME), Hamburg, Germany
Do your drug discovery workflows run like clockwork?
Let automated liquid handling ensure they do. Discover more in this infographic.
References
1. MacDonald A. Stem cells in drug discovery. Technology Networks April 4, 2017. Available at: https://www.technologynetworks.com/cell-science/articles/stem-cells-in-drug-discovery-286825 (Last accessed April 2018).
2. iPS Cell Drug Discovery Taking Off with First Clinical Trial. Press release. August 2, 2017. Available at: https://asia.nikkei.com/tech-science/science/ips-cell-drug-discovery-taking-off-with-first-clinical-trial (Last accessed April 2018).
3. Hwang TJ, Carpenter D, Lauffenburger JC, et al. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern Med 2016;176(12):1826-1833.
4. Onakpoya et al. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Medicine 2016; 14:10; DOI 10.1186/s12916-016-0553-2.
5. Yan, Papoian T. Moving beyond the comprehensive in vitro proarrhythmia assay: Use of human-induced pluripotent stem cell-derived cardiomyocytes to assess contractile effects associated with drug-induced structural cardiotoxicity. J Appl Toxicol 2017;doi:10.1002/jat.3611
About the author

Kevin Moore
Kevin Moore is Head of Markets and Applications based out of Tecan’s head office in Männedorf, Switzerland. He heads the team tasked with bringing both products and application for the liquid handling to the market. Prior to joining Tecan in 2007, he was head of Compound Management and Technology project manager for the Neuroscience Research Centre of Merck & Co in the UK, where he worked for Merck for 20 years.











