By Michael Fejtl
“When you can measure what you are speaking about, and express it in numbers, you know something about it.” Lord Kelvin knew that. To be confident in your results, to quickly move your studies forward, and to be the first to publish your conclusions, you need to know that your numbers are right. The proof you need lies in reproducibility, and reproducibility in any cell-based assay starts with accurate cell counts.
Lord Kelvin (mathematician, physicist, engineer, 1824-1907) knew that measuring accurately was the key to reliable, publishable results.
With numbers, you know
Cells are intrinsic to proliferation, gene expression, and cytotoxicity studies. If your counts are consistent in cell culture QC, passaging, and seeding you’ve already made a big contribution towards minimizing variability. Accurate confluence measurements are vital to help ensure your cells are ready for your experiments, and they may even dictate whether you can proceed with downstream applications. Accurate viability counts are the cornerstone of any end-point or kinetic assays. Those counts become results that may govern your next steps, or even conclude a study.
It's not easy to get reproducible cell counts
Even small variations at each touch-point can accumulate to cause statistically significant differences across runs and throughout experiments. If the cell concentration in the hemocytometer is too low, then the number of cells in the counting field might not represent the actual concentration in the culture. If it’s too high, counting is difficult both because of the density of cells and cell aggregation. All of these can contribute to inconsistent data.
Experimental variations are rarely due to true human error – scientists are careful, meticulous people and nobody likes to repeat experiments unnecessarily. But even with the most stringent protocols and guidelines manual cell counting and confluence assessment still rely on individual interpretation. If there are a lot of cell clusters or debris, it can be especially difficult to get consistent results.
As sources of variation multiply, you may no longer be able to identify the cause. It becomes incredibly difficult to measure and normalize cell count differences from well to well, across plates, and among experiments. If cell counting or confluence assessment is inconsistent your data may be skewed, your results unreliable, and your conclusions cloudy. You have to repeat experiments. You’ve lost time and wasted money.
It's a matter of trust
But with statistically significant data your results are clear. You have an unassailable audit system. You’re more confident in your interpretations and can finalize your conclusions. The path to your next steps is clear and you can complete your studies sooner.
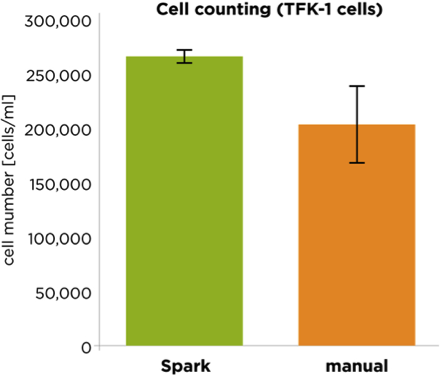
Automated cell counting yields greater accuracy and precision than manual cell counting with a hemocytometer.
Why is your lab still counting cells with hemocytometers and checking confluence with a microscope? Focus on the true variability that leads to exciting insights from your experiments. Don’t add unnecessary variability from cell culture, confluence, and viability counting. Automate your cell-based assays and trust your numbers.
About the author

Michael Fejtl
Michael Fejtl is a Market & Product Manager responsible for the multi-mode reader portfolio at Tecan Austria. Michael gained his PhD in Neurobiology at the University of Vienna. He then spent six years as a research scientist in the US. Following this he joined a university as head of electro-physiology, looking at multi-electrode array issues. Next, Michael entered the pharma market for automated patch clamping before working with micro plate readers and developed a special interest in cell-based assays. Michael joined Tecan in 2012.